[ad_1]
For sufferers with severe diseases, timeline entry to efficacious drugs is paramount. The European Medicines Company (EMA) was created partly to assist expedite drug approvals and insure these merchandise are save and efficient. As acknowledged in a paper by Grünwald and Stargardt (2024):
The EMA [European Medicines Agency] was based in 1995 primarily to harmonize the advertising authorization of prescribed drugs within the EU and EEA…as there had been substantial variations amongst European nations when it comes to launch delay and the supply of prescribed drugs
The EMA had 3 key group procedures that grant entry to the markets of some or all EU member nations concurrently.
- Centralized process (CP). If the EMA evaluates a pharmaceutical and grants it advertising authorization, this dedication is binding all all European Union member states. CP was launched in 1995 and was initially used just for “biotechnological processes, corresponding to monoclonal antibodies, managed gene expression or recombinant DNA expertise”. The checklist of treamtents evaluated below CP has expanded to includeorphan medication and substances in opposition to most cancers, diabetes, and HIV/AIDS (in 2005), viral ailments and auto-immune ailments/dysfunctions (in 2008), and superior remedy medicinal merchandise (e.g., cell and gene-therapy) additionally in 2008.
- Mutual recognition process (MRP). On this case, the evaluation is carried out by a reference member state, which the applicant can select freely and whose determination is subsequently adopted by all different member states through which the applicant seeks market entry. This process was adopted in 2001, and contains new remedies which are exterior of the CP corresponding to different prescribed drugs and generics.
- Decentralized process (DCP). Adopted in 2005, this might enable pharmaceutical producers to hunt nation by nation approval. That is solely eligible for brand spanking new substances not ruled by CP or MRP.
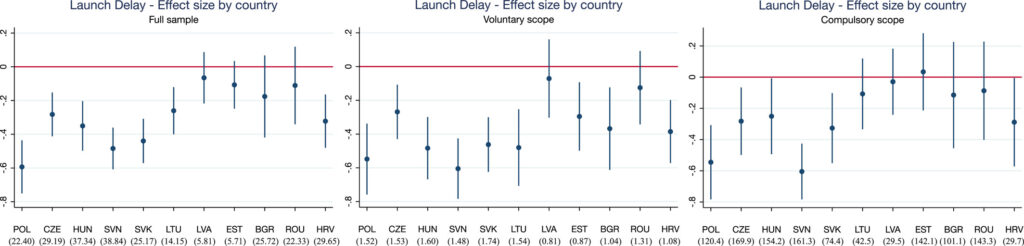
To look at the affect of those procedures, Grünwald and Stargardt (2024) conduct a differences-in-differences evaluation evaluating nations topic to those group procedures in opposition to those that weren’t. Particularly, with EU enlargement, in 2004 the Czech Republic, Estonia, Hungary, Latvia, Lithuania, Poland, Slovakia and Slovenia (Cyprus and Malta additionally joined the EU on this date however the authors didn’t have knowledge from these nations). In 2007, Bulgaria and Romania joined the EU after which Croatia joined in 2013. In distinction, Belarus, Bosnia and Herzegovina, Kazakhstan, Russia, Serbia, Switzerland , and Turkey by no means joined the EU. Utilizing IQVIA gross sales knowledge from 33 European nations, the authors examined (i) the launch delay and (ii) the supply of latest lively substances. The authors discover that,
…nations skilled a imply lower in launch delay of 10.9 months (p = 0.004) after becoming a member of the EU. Results had been greater amongst prescribed drugs that belong to indications which may voluntarily take part within the CP however aren’t obliged to. These are sometimes financially much less engaging to producers than prescribed drugs inside the obligatory scope. Availability of latest prescribed drugs launched remained unaffected. We discovered indicators that the magnitude of the country-specific impact of centralized advertising authorization on launch delay could also be influenced by strategic choices of producers on the nationwide degree (e.g., parallel commerce or reference pricing).
For extra particulars, you’ll be able to learn the total article right here.
[ad_2]

