[ad_1]
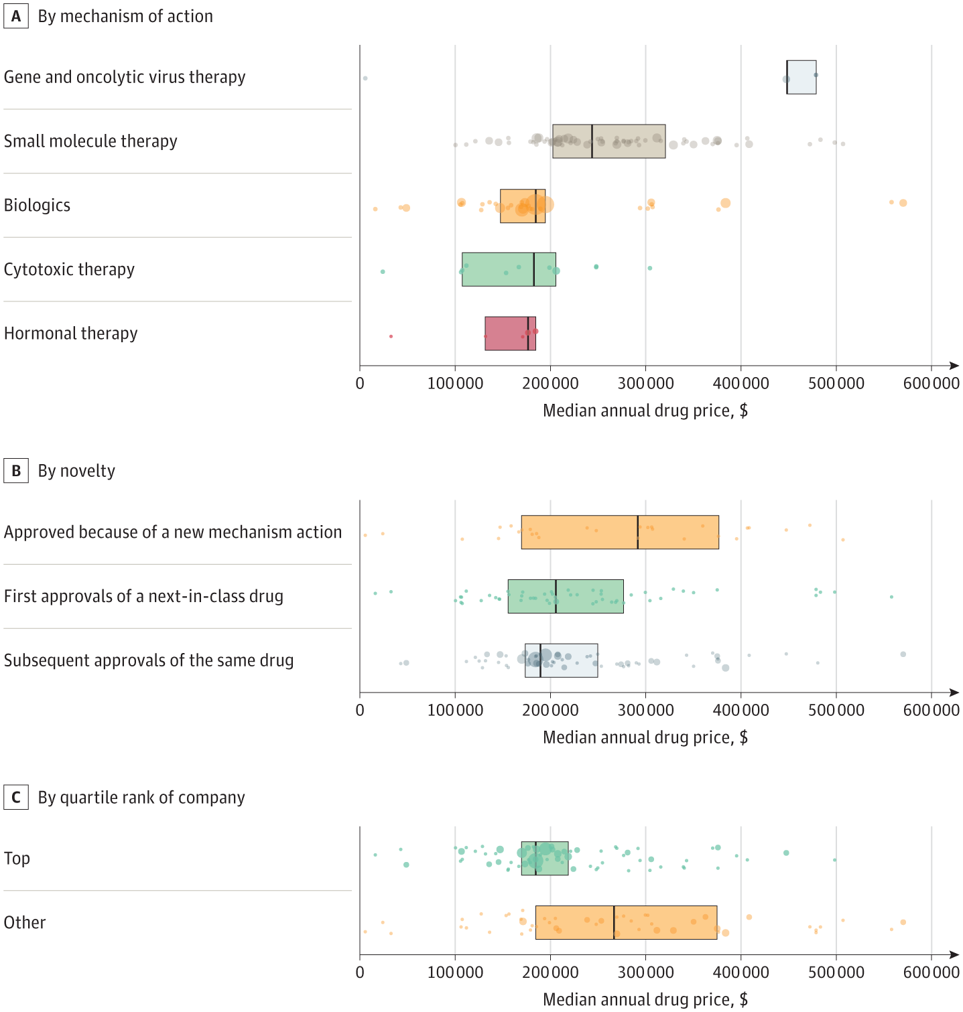
Based on a paper by Miljković et al. (2023), the reply is ‘no‘. The authors study oncology therapies with FDA approvals between 2015 and 2020 and determine common wholesale costs from Redbook. Medicine had been labeled into three classes: (i) first approval of a brand new mechanism of motion compound, (ii) next-in-class approval regardless the tumor kind, and (iii) subsequent approval of the identical drug. Utilizing this method, they discovered that:
There have been 224 most cancers drug approvals throughout 119 particular person medicine, with a median annual value of $196 000 (IQR, $170 000-$277 000). Gene and viral therapies had been the most costly (median, $448 000 [IQR, $448 000-$479 000]), adopted by small molecule remedy (median, $244 000 [IQR, $203 000-$321 000), and biologics (median, $185 000 [IQR, $148 000-$195 000]). There was no vital distinction in value between first-in-class, next-in-class, and subsequent approvals of an already authorized drug.
The total article is right here.
[ad_2]

