[ad_1]
After we consider utilization administration (e.g., prior authorizations, step edits), we regularly suppose payers solely use these for greater value branded merchandise together with biologics. Generic medicine ought to have low value sharing and restricted utilization administration. One query, nevertheless, is whether or not payers’ utilization administration practices for biosimilars mirror these of biologic merchandise, or small-molecule generics, or someplace in between.
A paper by Yu et al. (2023) goals to reply this query. The authors used knowledge from the Tufts Medical Heart Specialty Drug Proof and Protection (SPEC) database protecting 19 commercially-available biosimilars equivalent to 7 reference merchandise. These merchandise had been used for 28 distinctive indications. The authors discover that:
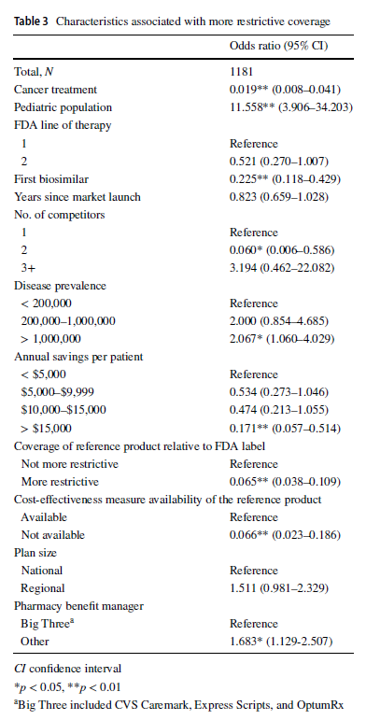
In contrast with reference merchandise, well being plans imposed protection exclusions or step remedy restrictions on biosimilars in 229 (19.4%) choices. Plans had been extra more likely to prohibit biosimilar protection for the pediatric inhabitants (odds ratio [OR] 11.558, 95% confidence interval [CI] 3.906–34.203), in ailments with US prevalence greater than 1,000,000 (OR 2.067, 95% CI 1.060–4.029), and if the plan didn’t contract with one of many three main pharmacy profit managers (OR 1.683, 95% CI 1.129–2.507). In contrast with the reference product, plans had been much less more likely to impose restrictions on the biosimilar–indication pairs if the biosimilar was indicated for most cancers therapies (OR 0.019, 95% CI 0.008–0.041), if the product was the primary biosimilar (OR 0.225, 95% CI 0.118–0.429), if the biosimilar had two rivals (reference product included; OR 0.060, 95% CI 0.006–0.586), if the biosimilar might generate annual checklist value financial savings of greater than $15,000 per affected person (OR 0.171, 95% CI 0.057–0.514), if the biosimilar’s reference product was restricted by the plan (OR 0.065, 95% CI 0.038–0.109), or if a cost-effectiveness measure was not obtainable (OR 0.066, 95% CI 0.023–0.186).
One attention-grabbing discovering was that giant PBMs truly had much less restrictive insurance policies over biosimilars. Why?
… it has been posited that the bargaining energy of bigger PBMs could also be so important that biosimilar producers could typically increase checklist costs, and therefore rebates, to acquire a spot on the formularies of enormous PBMs. This would go away smaller PBMs with greater checklist costs however
smaller rebates resulting from their comparatively smaller bargaining energy, wherein case the biosimilars deliver much less worth to them.
You may learn the total paper right here.
[ad_2]

